Bracco Reimbursement Hotline: 1-800-349-1388
askbracco@reimbursement.bracco.com
Coding in Echocardiography with an Enhancing Agent
Scenario 1 – HOPPS, Commercially Insured
Scenario 2 – HOPPS, Medicare
Scenario 3 – IDTF, Medicare
Scenario 4 – IDTF, Commercially Insured
Bracco Reimbursement and Health Policy Support
Navigating Reimbursement
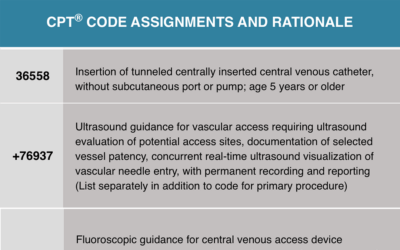
Venous Coding Case Study¹
Case studies are great learning tools for coders, providing an array of detail and rationale to enhance understanding. Here, we explore a case study related to venous access for correct coding. By examining case studies like these, coders can ensure success throughout...
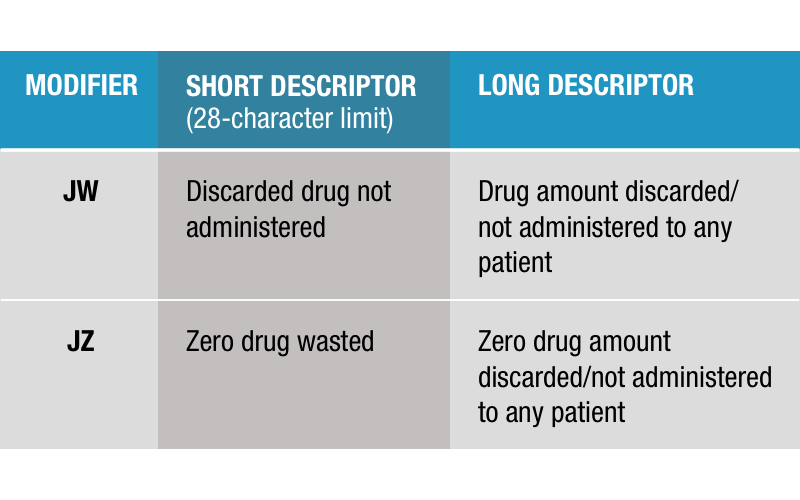
Introducing the JZ Modifier
In the 2023 Centers for Medicare & Medicaid Services (CMS) Physician Fee Schedule Final Rule, CMS confirmed the implementation of the new JZ Modifier. The JZ Modifier became available to use as of January 1, 2023, and is required to be implemented no later than...
2023 CMS Final Rules HOPPS and MPFS
CMS has finalized the rules for the 2023 Hospital Outpatient Prospective Payment System (HOPPS) and Medicare Physician Fee Schedule (MPFS). Please see below for some key information impacting diagnostic imaging payments for 2023. For more information on the HOPPS...
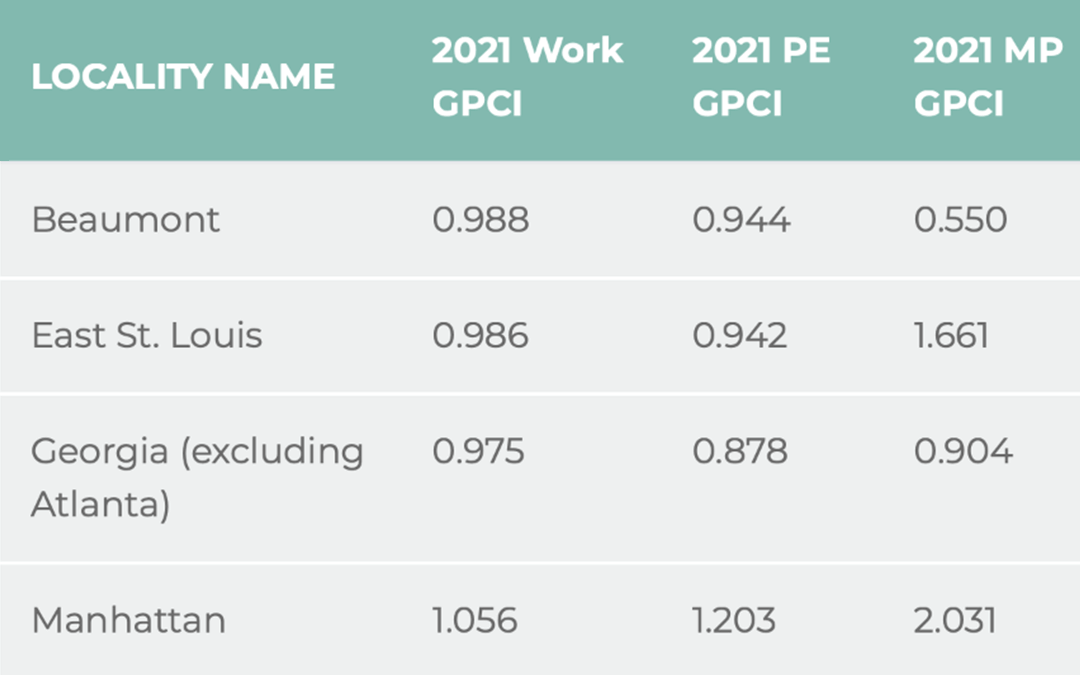
Relative Value Units
RVUs are the basic component of the Resource-Based Relative Value Scale (RBRVS), which is a methodology used by CMS and private payers to determine physician payment. RVUs are not the sole factor that defines physician compensation: the physician payment is determined...
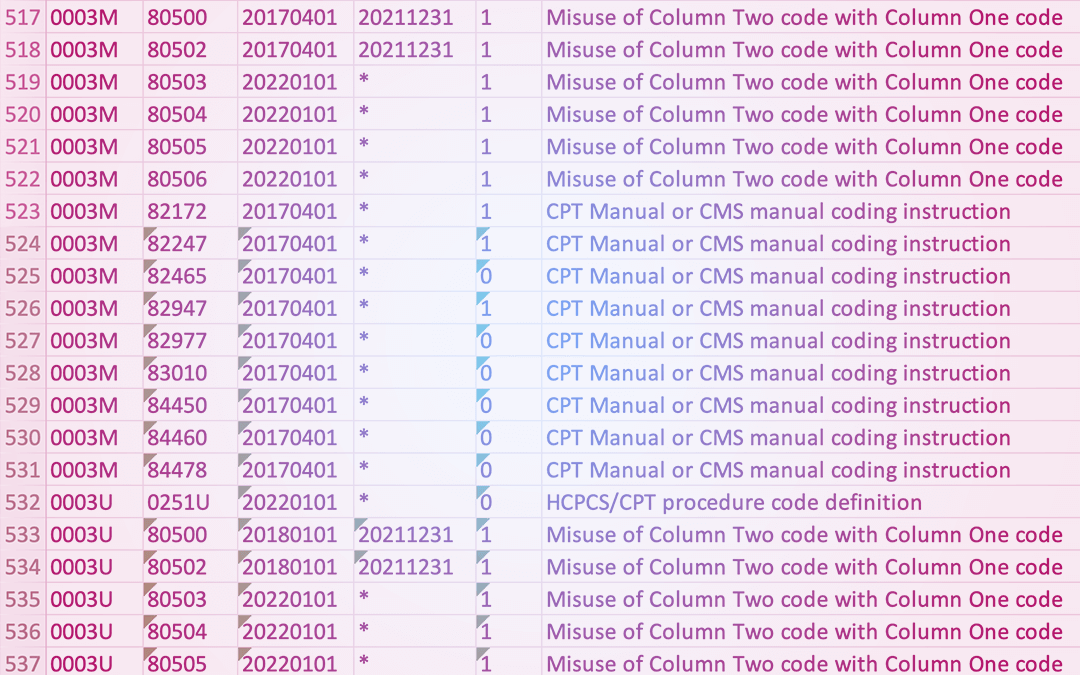
National Correct Coding Initiative (NCCI) Edits
CMS has developed coding edits in order to prevent improper payments. There are three different types of edits. Procedure to Procedure (PTP) edits prevent improper payment when incorrect code combinations are reported. Medically Unlikely Edits (MUE) are to prevent...
Radiology Benefit Managers (RBMs)
As a personal consumer of healthcare, you may be quite familiar with your Pharmacy Benefit Manager (PBM). When going to the pharmacy to pick up your prescription (Part D) drug, in order to have your your prescription covered by insurance you are asked to provide proof...
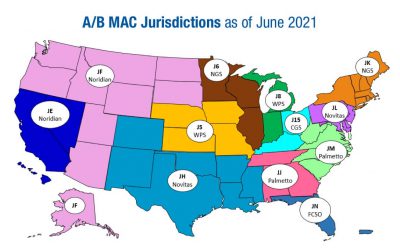
Medicare Administrative Contractors
A Medicare Administrative Contractor (MAC) is a private health care insurer that has been awarded a geographic jurisdiction to process Medicare medical claims for Medicare Fee-For-Service (FFS) beneficiaries. CMS relies on a network of MACs to serve as the primary...
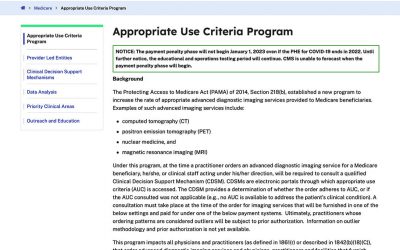
Appropriate Use Criteria
As described by the Centers for Medicare and Medicaid Services (CMS), the Protecting Access to Medicare Act (PAMA) of 2014, Section 218(b), established a new program to increase the rate of appropriate advanced diagnostic imaging services provided to Medicare...
Hospital Price Transparency
Beginning January 1, 2022, CMS increased the penalty for some hospitals that do not comply with the Hospital Price Transparency final rule. However, it has been noted that thousands of hospitals are not in compliance. Click here to learn more.
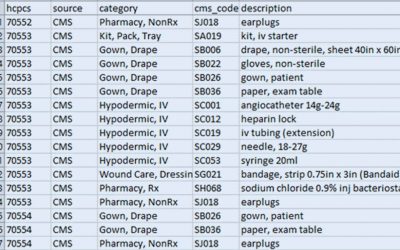
NDC/HCPCS Crosswalk Files
Finding the Healthcare Common Procedure Coding System (HCPCS) code for a new product or drug is easier than you may think! The Centers for Medicare & Medicaid Services (CMS) publishes an updated National Drug Code (NDC)/HCPCS crosswalk file every quarter. You can...
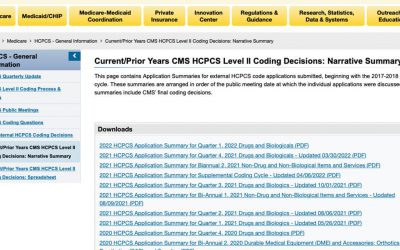
HCPCS Coding Decisions
Since 2020, the Centers for Medicare & Medicaid Services (CMS) has been reviewing Healthcare Common Procedure Coding System (HCPCS) applications quarterly and posting their decisions with explanations on their website. To access the HCPCS application summaries,...
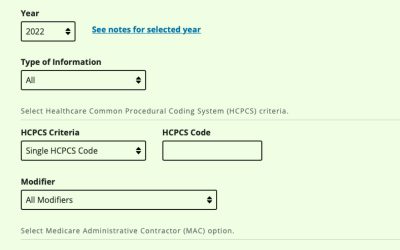
Physician Fee Schedule Lookup Tool
Did you know that Medicare makes reimbursement rates for procedures done in physician's offices public information? To look up procedure reimbursement rates in your area or for the National Payment Amount, check out the Centers for Medicare & Medicaid Services...
Procedure Input Files
Under Medicare, the components for many procedures are “bundled” together under one code. The bundle includes important components of the procedure, such as: staff time, disposable plastics, electricity for the building and—under the Hospital Outpatient Prospective...
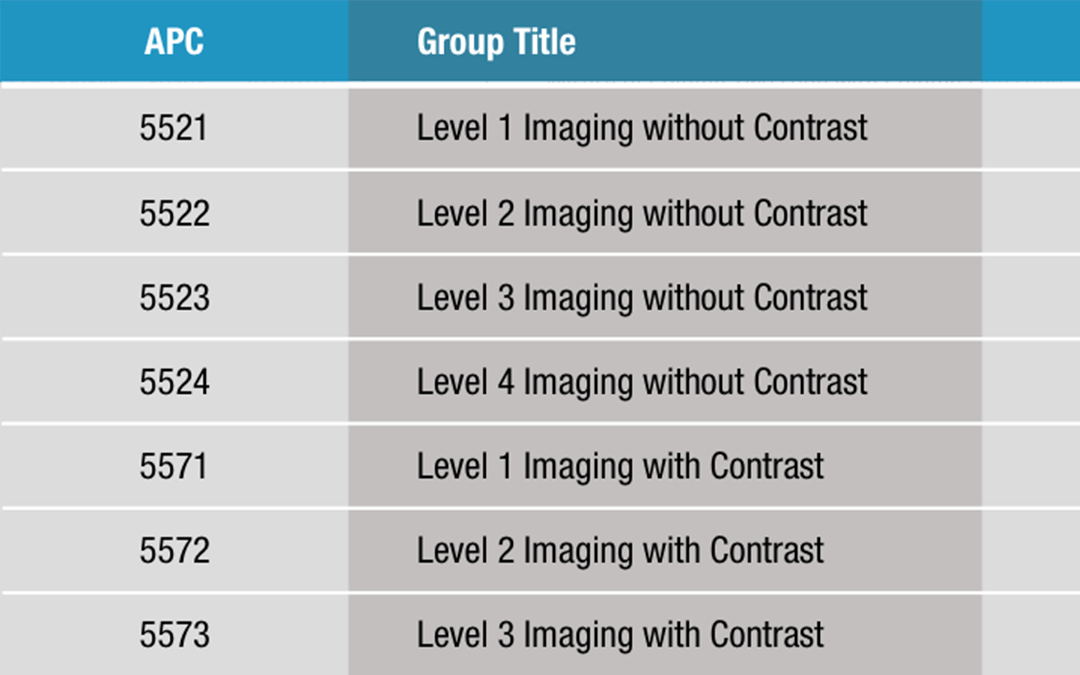
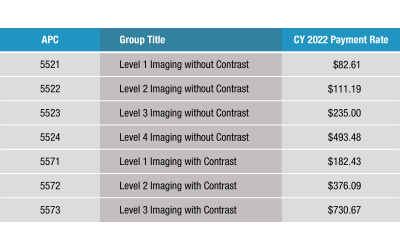
Ambulatory Payment Classifications (APCs)
The Centers for Medicare and Medicaid Services (CMS) has developed Ambulatory Payment Classifications (APCs) as a method of payment for hospital outpatient services. Each APC is composed of procedures which are similar in clinical intensity, resource utilization, and...
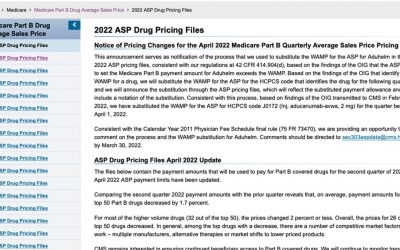
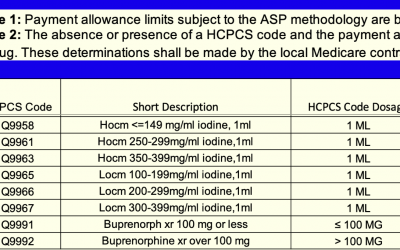
ASP Pricing Files
The Centers for Medicare & Medicaid Services (CMS) Medicare program reimburses for Part B drugs based on the Average Sales Price (ASP) of a drug +6%. These ASP rates are submitted quarterly by drug manufacturers and are based on data from two quarters prior.¹...